An interdisciplinary approach to study the neural circuit basis of motivated behavior
The Stuber lab is located on the 1st floor of the J-wing Health Science Building at the University of Washington as is part of the UW NAPE Center. The lab space consists of ~4,000 square feet of main space equipped with desks and state of the art computers, as well as a wet lab for solution preparation, brain slice preparation, stereotaxic surgery, histology, optical fiber and electrode preparation, and molecular biology.
Approaches and Techniques
Optogenetics
In situ hybridization
Immunohistochemistry
Behavioral assays
In vivo calcium imaging
Single cell transcriptional profiling
Confocal & multiphoton microscopy
Brain slice electrophysiology
In vivo electrophysiology
Major Lab Equipment
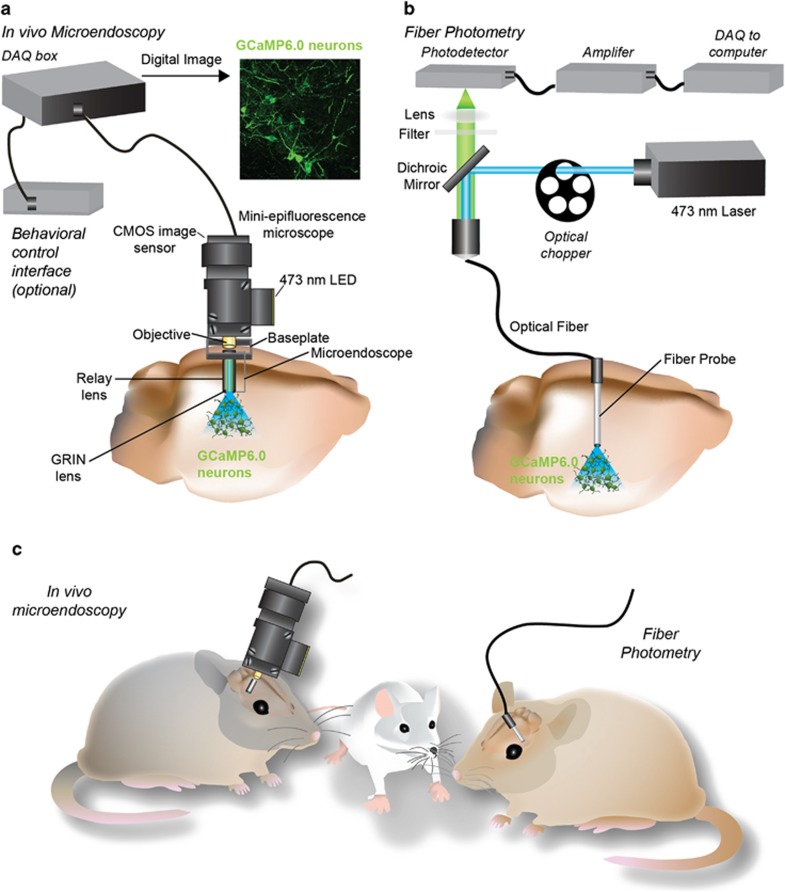
Three systems for in vivo calcium imaging during behavior. These systems were obtained from Inscopix through their early access program, which aims to transfer this technology to neuroscience research labs that have extensive experience in studying neural circuit function. These miniature endoscopic systems utilize implantable GRIN lenses for optical imaging, and we have successfully used this approach to image the activity dynamics of 100s of neurons simultaneously in freely behaving mice. We are continuing to work closely with the scientists at Inscopix to further develop and optimize this approach for the use in sophisticated behavioral experiments.
Two video tracking systems with custom built optogenetic hardware. These setups utilize Ethovision (Noldus Technologies) for real-time tracking of mouse behavior. This includes the additional three point tracking module for measuring social behavior. Both of these systems are equipped with custom built optogenetic hardware for precise neural circuit manipulations.
Two brain slice electrophysiology rigs. Each rig is equipped with an Olympus BX51 microscopes with optics for differential interference contrast (DIC) and fluorescent imaging. Each rig also includes a two-channel low noise amplifier with relevant accessories for patch-clamp recordings in brain slices, and LEDs tuned to various wavelengths for photostimulation or uncaging of compounds as well as the activation of optically excitable genetically engineered proteins, such as ChR2.
Eight mouse operant chambers equipped with optogenetic hardware. These are used for behavioral testing of transgenic and wild type mice. These chambers are also equipped with custom-built optogenetic hardware for in vivo circuit manipulation with millisecond precision.
A 32 channel in vivo electrophysiology system equipped with optogenetic hardware. This is a system from Plexon Instruments used for monitoring changes in single unit activity and local field potentials during behavior. This system is interfaced with a 32 channel motorized commutator interfaced with an optical commutator and laser-based fiber-optics to perform optogenetic photo-tagging coupled with electrophysiology.
Three stereotaxic surgical stations with dissecting optics and virus delivery systems. One of these surgical stations is also equipped with a motorized micromanipulator for GRIN lens implantation surgeries and installation of the endoscopic baseplates. This system also is also equipped for in vivo anesthetized electrophysiology recording.
A resonant-scanning multi-photon microscope for in vivo imaging. We purchase an Olympus Fluoview FVMPE-RS microscope and a Titanium Sapphire laser with dispersion compensation (MaiTai DeepSee EPS). This system will is used for in vivo and brain slice multi-photon calcium imaging as well as detailed structural analysis of intact neural tissue.